Doctors prescribe generics every day. In fact, 90% of all prescriptions filled in the U.S. are for generic medications. Yet, many clinicians still hesitate-sometimes because they’re unsure if generics are truly equivalent, or because they’ve heard patient concerns that don’t match the science. The truth is, generics aren’t just cheaper-they’re clinically identical. But getting that message across isn’t easy. That’s why structured prescriber education resources exist: to give doctors clear, evidence-based tools to understand, trust, and confidently recommend generics.
What Makes a Generic Drug the Same as a Brand-Name Drug?
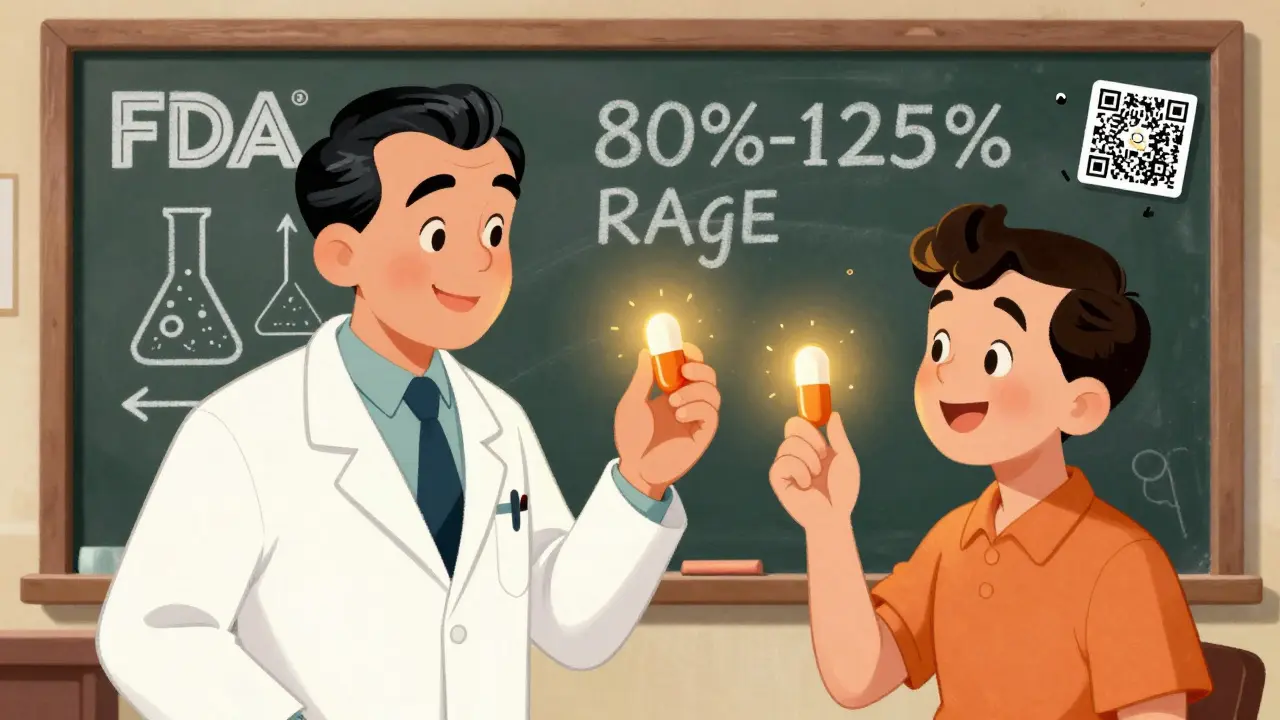
The FDA doesn’t approve generics based on guesswork. Every generic must pass strict testing to prove it works the same way as the brand-name version. The key standard? Bioequivalence. This means the generic must deliver the same amount of active ingredient into the bloodstream at the same rate as the brand. The FDA requires this to happen within an 80% to 125% range. That’s not a wide gap-it’s a tight window. For example, if a brand-name pill delivers 100 units of medicine, the generic must deliver between 80 and 125 units. Most generics land right in the middle, often within 98% to 102%.
These aren’t lab curiosities. Each generic goes through testing in 24 to 36 healthy volunteers. The data is reviewed by FDA scientists before approval. Once approved, the generic is monitored just like the brand. In 2022, the FDA analyzed over 12,000 adverse event reports for generics and nearly 12,000 for brand-name drugs. The numbers were nearly identical. No hidden risks. No hidden differences.
Why Do Some Doctors Still Doubt Generics?
It’s not about science-it’s about perception. Some doctors remember when generics were less reliable, decades ago. Others have patients who swear the brand works better. A 2021 FDA survey found that 42% of Hispanic patients and 38% of patients with household incomes under $25,000 believed generics were less effective. These beliefs don’t come from nowhere. They come from misinformation, confusing packaging, or even well-meaning but outdated advice.
One major issue? Lack of exposure to current data. A 2022 study found only 48% of physicians knew about the FDA’s Generic Drugs Stakeholder Toolkit, even though it’s been available since 2019. Many doctors never see these resources because they’re not built into their workflow. A PDF handout on a shelf doesn’t help when you’re rushing between patients. What works better? When the information is right where you need it-inside your electronic health record.
How the FDA’s Resources Are Designed for Real Clinics
The FDA didn’t just dump data online. They built tools for daily use. The Prescriber Flyer (Version 2, updated in March 2022) is a single page, designed to fit in a waiting room rack. It includes a QR code linking to Spanish-language materials, addressing health equity gaps. The Generic Drugs and Health Equity Handout uses simple language-written at a 6th to 8th grade reading level-and shows real numbers: patients with low incomes are 3.7 times more likely to stop taking their meds because of cost.
The toolkit also includes five customizable information cards. These can be printed and handed to patients during a visit. One card explains the approval process in five steps: active ingredient, dosage, strength, route of administration, and bioequivalence testing. Another compares manufacturing standards-brand and generic drugs are made in the same facilities, under the same rules. The FDA even shares that 40% of brand-name drugs are made in the same plants as generics.
There’s also a visual infographic titled What Makes a Generic the Same as a Brand-Name Drug? It shows side-by-side comparisons of how both types of drugs are tested, produced, and monitored. No jargon. No charts that require a PhD. Just clear, visual proof.

What Other Organizations Are Doing
The American College of Physicians (ACP) took a bold stance in 2015: “All clinicians should prescribe generic medications whenever possible.” Their reasoning? Cost drives non-adherence. About 20% to 30% of new prescriptions are never filled because patients can’t afford them. A $300/month brand-name drug can drop to $37.50 as a generic. That’s $262.50 saved every month. For a diabetic patient on insulin, that difference can mean the difference between managing their condition and ending up in the ER.
The CDC, in its 2022 Opioid Prescribing Guideline, noted that 78% of opioid prescriptions could be switched to generics without losing effectiveness. They didn’t just mention it-they embedded the idea into clinical guidelines for pain management.
In Europe, the EMA takes a slightly different approach. Instead of focusing only on blood levels, they also compare how the drug dissolves in the body. But here’s the key: despite these minor differences, 95% of scientific standards between the U.S. and EU align. The goal is the same: ensure safety and effectiveness.
Where Education Falls Short-and How to Fix It
Most doctors agree generics are safe. But many still don’t use them as often as they could. Why? Time. A 2022 study in Annals of Internal Medicine found 73% of physicians said they didn’t have time to review educational materials during appointments. Another survey found 68% of doctors found the FDA flyers useful but too technical to use quickly.
The fix? Integration. Kaiser Permanente didn’t just hand out brochures. They put FDA-approved generic information directly into their Epic EHR system. When a doctor typed in a brand-name drug, a pop-up appeared: “Generic available. Saves patient $262/month.” Within six months, brand prescribing dropped by 18.7%.
Other successful models include:
- 15-minute training sessions during staff meetings, explaining bioequivalence and patient communication scripts
- Adding prompts to intake forms: “Do you have concerns about switching to a generic?”
- Monthly feedback reports showing how a doctor’s generic prescribing rate compares to peers
One rural family physician in Nebraska increased her generic prescribing rate from 62% to 89% in 18 months-just by using the FDA infographic during patient visits. She told patients: “This is the same medicine. Made in the same factory. Tested the same way. The only difference? The price.”
The Future: AI, EHRs, and Personalized Education
The next wave of education is digital and personalized. In July 2023, the FDA launched a pilot program connecting its generic drug database directly to Epic and Cerner systems via API. Early results? A 15.2% increase in generic prescribing among participating doctors in just six months.
IBM Watson Health tested an AI tool that analyzed patient records and generated personalized messages. For example, if a patient had a history of non-adherence due to cost, the system would suggest: “Switching to this generic saves $250/month. 99.7% of patients report no change in effectiveness.” In a trial with 120 doctors, patient acceptance jumped by 29 percentage points.
And it’s not just tech. Medicare Part D is now considering financial incentives for plans that educate prescribers on therapeutic alternatives. That means hospitals and insurers will soon have a reason to invest in this training.
What Doctors Need to Know Now
You don’t need to be a pharmacologist to prescribe generics confidently. Here’s what matters:
- Generics are bioequivalent. They deliver the same active ingredient at the same rate. The 80-125% range isn’t a loophole-it’s a scientifically validated standard.
- They’re made under the same rules. The FDA inspects generic manufacturing plants as often as brand-name ones.
- They’re safer than you think. Adverse event reports for generics and brands are nearly identical.
- Cost saves lives. Patients who can’t afford their meds stop taking them. Generics reduce non-adherence by up to 40%.
- Patients need clear explanations. Use simple language: “It’s the same medicine. Just cheaper.”
And if you’re unsure? The FDA’s resources are free, updated, and built for clinicians. Download the Prescriber Flyer. Print the infographic. Add the conversation prompts to your workflow. You don’t need to memorize the ANDA process. You just need to know it works-and help your patients understand why.
Are generic drugs really as effective as brand-name drugs?
Yes. Every generic drug must meet the FDA’s strict bioequivalence standards, meaning it delivers the same active ingredient at the same rate and amount as the brand-name version. Testing involves 24-36 healthy volunteers and measures blood concentration over time. The acceptable range is 80% to 125%-most generics fall within 98% to 102%. Over 90% of prescriptions in the U.S. are for generics, and adverse event reports show no meaningful difference in safety.
Why do some patients believe generics don’t work as well?
Misconceptions often come from packaging differences, outdated experiences, or misinformation. Some patients remember when generics were less consistent decades ago. Others confuse “authorized generics” (same drug, different packaging) with true generics. Language barriers and cost-related anxiety also play a role-42% of Hispanic patients and many low-income patients express concerns about quality. Clear, visual explanations using FDA tools can reduce these fears by showing identical manufacturing and testing standards.
Can I trust generics made overseas?
Yes. The FDA inspects all manufacturing facilities-whether in the U.S., India, China, or elsewhere-using the same standards. About 40% of brand-name drugs are also made overseas. The FDA conducts over 3,500 inspections annually, with 90% of facilities passing. A generic made abroad meets the same purity, strength, and quality requirements as one made in the U.S. The location doesn’t determine safety-the inspection record does.
Do generics have different side effects?
No. The active ingredient is identical, so side effects are the same. Differences in inactive ingredients (like fillers or dyes) may cause rare allergic reactions in sensitive individuals, but these are not related to effectiveness. The FDA reviews all ingredients before approval. If a patient reports a reaction, switching to another generic or the brand may help-but it’s not because the generic is less safe.
How can I start using these resources in my practice?
Start simple. Download the FDA’s Prescriber Flyer and Generic Drug Facts Handout. Print them and place them where patients can see them. Use the infographic during visits to show how generics are tested. Ask your EHR vendor if they integrate FDA data. If not, advocate for it. Begin with one condition-like hypertension or diabetes-and track how many patients switch to generics after you explain the benefits. Even small changes add up: physicians who received structured education were 2.3 times more likely to discuss cost with patients.
What’s Next?
The data is clear: generics save money, improve adherence, and work just as well. The gap isn’t in science-it’s in access to information. Doctors who use the FDA’s tools see higher generic prescribing rates. Patients who understand the facts are more willing to switch. The next step? Make these resources part of your daily routine. Not as a handout. Not as a PDF. As a conversation. Because the best education isn’t a lecture-it’s a moment of clarity in the exam room.



Benjamin Fox
February 18, 2026 AT 15:48