Switching from a brand-name drug to a generic version seems like a simple way to save money. But for some medications, that swap isn’t harmless-it can be dangerous. This is especially true for NTI drugs, or Narrow Therapeutic Index drugs. These are medicines where the difference between a safe, effective dose and a toxic one is razor-thin. Even tiny changes in how your body absorbs the drug can push you into danger.
What Makes a Drug an NTI Drug?
NTI drugs have a very narrow window between helping you and hurting you. The FDA defines them as medications where small changes in dose or blood concentration can cause serious side effects-or make the drug stop working entirely. Think of it like walking a tightrope. One small misstep, and you fall.
Take warfarin, for example. It’s used to prevent blood clots, but if your blood levels dip too low, you could get a stroke. If they rise too high, you could bleed internally. The target range is so tight-your INR must stay between 2.0 and 3.0-that even a 10% shift in drug absorption can throw you out of safety.
Phenytoin, used for seizures, has a similar problem. The effective range is 10 to 20 mcg/mL. Go above 20, and you risk dizziness, uncontrolled eye movements, and even coma. Go below 10, and you could have a breakthrough seizure. There’s no room for error.
Other common NTI drugs include digoxin (for heart rhythm), lithium (for bipolar disorder), and theophylline (for asthma). Even methadone, used for pain and opioid addiction, falls into this category. In someone who hasn’t built up tolerance, the dose that relieves pain is almost the same as the dose that stops breathing.
Why Generic Switching Is Risky
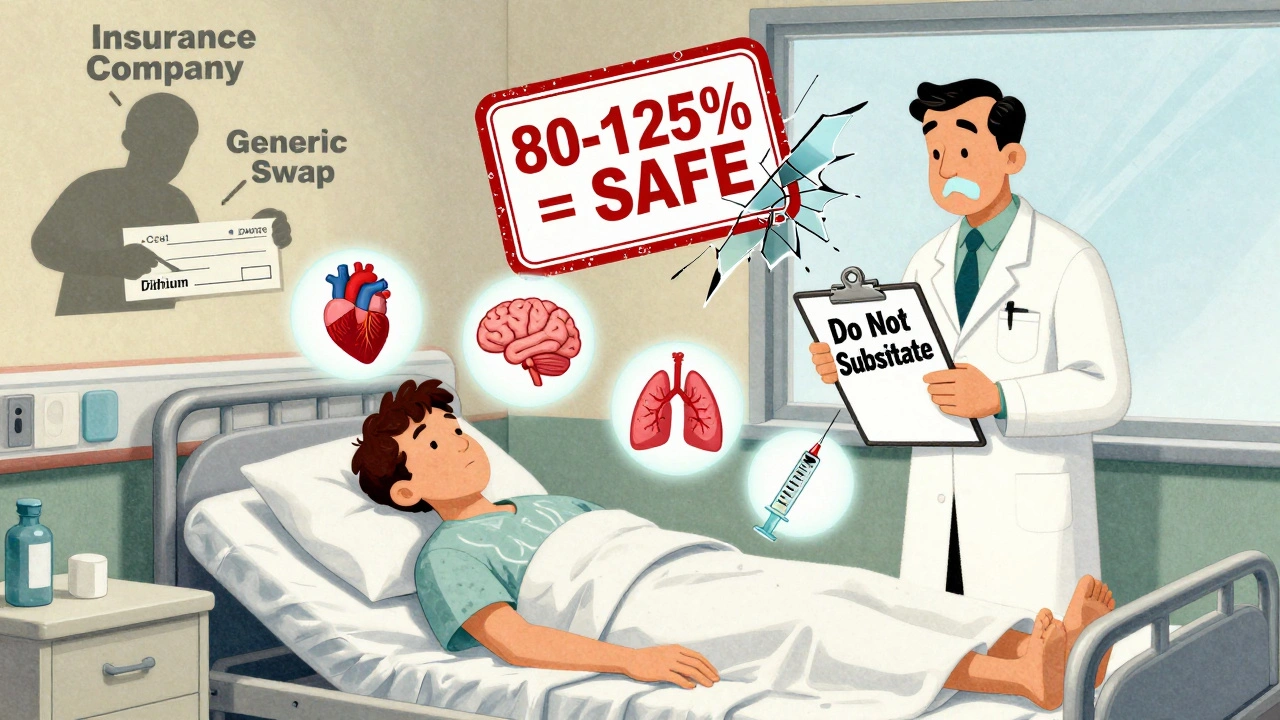
Generic drugs are required to be bioequivalent to the brand-name version. That means they must deliver 80% to 125% of the same amount of drug into your bloodstream as the original. Sounds reasonable, right? But for NTI drugs, that 45% swing is too wide.
Let’s say your brand-name warfarin gives you a blood level of 1.8 mcg/mL-perfect for your INR. You switch to a generic that delivers 125% of that amount. Suddenly, your level jumps to 2.25 mcg/mL. You’re now outside the safe range. No warning. No symptoms at first. But your risk of bleeding skyrockets.
It’s not just about the total amount. Some generics are absorbed differently depending on food, stomach pH, or even the time of day you take them. Phenytoin is notorious for this. One study found patients who switched to a different generic version had unpredictable spikes and drops in blood levels-even though both generics were technically "bioequivalent."
Real Cases, Real Consequences
This isn’t theoretical. In the 1980s, patients on phenytoin started having seizures after switching to a cheaper generic. In one case, a man had a grand mal seizure just days after his pharmacy switched his prescription without telling him. His blood level had dropped below 10 mcg/mL. He wasn’t told to get tested. He wasn’t warned.
With warfarin, multiple studies have shown INR levels shifting after switching from Coumadin to generic warfarin. Some patients stayed stable. Others didn’t. One study found patients needed more frequent blood tests after switching-not because they were doing anything differently, but because the generic behaved differently in their bodies.
Opioid users on methadone have reported sudden sedation or respiratory depression after switching to a generic with higher bioavailability. Others have suffered severe pain because the generic didn’t release enough drug. In both cases, the difference wasn’t obvious on paper. It only showed up in real people.

The FDA’s Stance vs. Reality
The FDA says generic NTI drugs are therapeutically equivalent. They’ve approved them. They say they work the same. But here’s the problem: the approval standard was never designed for drugs this sensitive.
In 2010, the FDA’s advisory committee concluded the 80-125% range was still acceptable for NTI drugs. But many experts disagree. The American Medical Association (AMA) has said for years that the decision to switch should be made by the doctor-not the pharmacist or the insurance company.
Some states, like North Carolina, have laws that restrict automatic substitution for NTI drugs. Pharmacists must get permission from the prescriber before swapping. Other states? No such rules. You could be switched without knowing it.
What Patients Need to Know
If you’re on an NTI drug, here’s what you must do:
- Know your medication. Is it an NTI drug? Ask your doctor or pharmacist. Common ones include warfarin, phenytoin, lithium, digoxin, theophylline, and methadone.
- Never let your pharmacy switch your drug without telling you. Ask if your prescription is being filled with the same brand or a generic.
- If you’re switched, ask for a blood test. For warfarin, check your INR within a week. For phenytoin or lithium, ask for a serum level test.
- Keep a written list of all your medications, including doses and when you started them. Share it with every doctor you see.
- Don’t assume generics are interchangeable. Even two different generics of the same drug can behave differently in your body.
Some patients worry about cost. Yes, generics are cheaper. But the cost of a hospital stay from a seizure, a stroke, or internal bleeding? That’s far higher.
What Doctors and Pharmacists Should Do
Doctors prescribing NTI drugs should specify "Do Not Substitute" on the prescription if they’re concerned. That legally blocks automatic switching in most states.
Pharmacists should be trained to recognize NTI drugs and flag any switch. They should offer to contact the prescriber before swapping. And they should document every substitution-especially for high-risk drugs.
There’s no universal list of NTI drugs. But if a drug requires regular blood monitoring, it’s probably on the list. If your doctor says, "We need to check your levels every few weeks," that’s a red flag.
The Bigger Picture
The push for generic drugs comes from a good place: lowering healthcare costs. But for NTI drugs, cost savings shouldn’t come at the cost of safety. The current bioequivalence standards were set for drugs like antibiotics or blood pressure pills-not for medications where a 10% change can kill.
Experts are calling for stricter standards. Some suggest lowering the bioequivalence range to 90-111% for NTI drugs. Others want mandatory therapeutic drug monitoring after any switch. The FDA has acknowledged the need for tighter limits-but hasn’t changed the rules yet.
Until then, the safest approach is simple: don’t switch unless your doctor says so. And if you’re switched without your knowledge, speak up. Your life might depend on it.
What does NTI mean in drugs?
NTI stands for Narrow Therapeutic Index. It means the drug has a very small window between the dose that works and the dose that causes harm. For example, with warfarin, a slight increase in blood level can cause dangerous bleeding, while a slight drop can lead to a clot. The ratio between toxic and effective dose is 2:1 or less.
Can I safely switch from brand-name warfarin to generic?
Some patients can switch without issues, but many can’t. Studies show that INR levels can change after switching, even if both versions are "bioequivalent." If you switch, get your INR checked within 3 to 7 days. Always talk to your doctor before making the change.
Which drugs are considered NTI drugs?
Common NTI drugs include warfarin, phenytoin, lithium, digoxin, theophylline, cyclosporine, and methadone. These drugs require regular blood tests to make sure levels stay in the safe range. If your doctor orders frequent lab work, your medication is likely an NTI drug.
Why doesn’t the FDA require stricter standards for NTI generics?
The FDA currently uses the same 80-125% bioequivalence standard for all generics, including NTI drugs. While experts and some advisory panels have urged tighter limits, no official change has been made. The agency says current data supports safety, but critics argue the data is outdated and doesn’t reflect real-world patient outcomes.
What should I do if my pharmacy switches my NTI drug without telling me?
Contact your doctor immediately. Ask for a blood test to check your drug level. If you’re on warfarin, get your INR checked right away. If you’re on phenytoin or lithium, request a serum level test. Report the switch to your doctor and ask them to write "Do Not Substitute" on future prescriptions.
Can I ask my doctor to prescribe only the brand-name NTI drug?
Yes. Your doctor can write "Dispense as Written" or "Do Not Substitute" on your prescription. This legally prevents the pharmacy from switching to a generic without your doctor’s approval. It’s your right to request this, especially for high-risk medications.

Donna Hammond
December 13, 2025 AT 21:04I was switched from Coumadin to generic warfarin last year without a word. My INR went from 2.4 to 3.8 in three days. I ended up in the ER with a massive bruise on my thigh from a cough. No one told me to monitor it. I’m lucky I didn’t bleed out. Now I demand "Do Not Substitute" on every script. Don’t let this happen to you.
Richard Ayres
December 14, 2025 AT 21:18The science behind bioequivalence thresholds is fundamentally misapplied to NTI drugs. The 80-125% range was designed for antibiotics and antihypertensives, not for medications where a 10% variance can induce coma or death. Regulatory bodies must acknowledge that not all drugs are created equal-and patient safety should override cost efficiency when the stakes are this high.
Jennifer Taylor
December 15, 2025 AT 23:23THE PHARMA COMPANIES ARE HIDING THIS ON PURPOSE. They know generics can kill. They pay off the FDA. I read a leaked memo once-there’s a secret meeting every year where they decide which drugs "can afford" to be switched. Lithium? They don’t care. Warfarin? "Eh, people will die, but we’ll save $200 million." I swear, if you look at the stock prices of the big generic makers right after a new NTI switch gets approved, you’ll see spikes. It’s not coincidence. It’s murder with a balance sheet.
Shelby Ume
December 16, 2025 AT 13:08Hey everyone-just wanted to say thank you for this thread. I’m a nurse in rural Ohio, and I see this every week. A patient comes in panicked because their seizures came back after a "routine" switch. They didn’t know they were switched. No one warned them. I keep a printed list of NTI drugs taped to my desk. I hand it out. I call pharmacies. I’ve saved lives just by asking, "Did you get the same brand?" It’s not complicated. It’s just not done. Let’s make it normal to ask.
Jade Hovet
December 18, 2025 AT 07:43OMG YES!! 🙌 I’m on lithium and my pharmacy switched me last month without telling me 😭 I felt like I was drunk for 3 days-slurred speech, shaky hands, felt like my brain was melting. Got my levels checked-yep, 1.5 instead of 0.8. Called my doc, they were furious too. Now I have a sticker on my pill bottle that says "NO SUBS!" and I show it to the pharmacist every time. You gotta be your own advocate!! 💪❤️
nina nakamura
December 19, 2025 AT 09:48Rawlson King
December 19, 2025 AT 17:40It’s not the generics. It’s the lack of monitoring. If patients were properly tested after every switch, none of this would matter. The real failure is in the healthcare system’s negligence-not the FDA’s standards. Stop blaming corporations. Blame the doctors who don’t follow up. Blame the pharmacists who don’t educate. Blame the patients who don’t ask questions. This is a systemic failure, not a regulatory one.
Tom Zerkoff
December 20, 2025 AT 06:49I want to commend Donna and Jade for sharing their experiences. That’s exactly the kind of real-world insight that policy makers need to hear. The data we have from clinical trials doesn’t capture the variability of human metabolism, diet, or even gut flora. Two people can take the exact same pill and have wildly different outcomes. That’s why we need mandatory post-switch lab testing for NTI drugs-and why pharmacists need to be trained to treat these switches like surgical procedures, not inventory changes. This isn’t about cost. It’s about dignity and safety.
kevin moranga
December 21, 2025 AT 21:06Look, I get it. I’ve been on phenytoin for 18 years. I’ve been switched three times. Twice I was fine. Once? I had a seizure in the grocery store. Didn’t even remember falling. My wife said I was staring blankly for 45 seconds. Turned out the new generic had a different filler-something about magnesium stearate changed how fast it dissolved. I didn’t know until I got my levels checked. Now I only take the brand. It costs $200 a month. I work two jobs to afford it. But I’m alive. And my kids have their dad. If you’re on an NTI drug? Don’t gamble. Ask for the brand. Write "Do Not Substitute" in bold letters. Make it a fight. Because your life? It’s worth more than a $10 savings.